Recently, the trend of ‘freezing honey’ has gained popularity on social media, which has sparked public interest in the physical properties of honey beyond a simple fad. Particularly, there has been a response that honey becomes harder when it is boiled and then placed in the freezer, which also increases interest in the scientific reasons behind this phenomenon.

While it may seem like ice at first glance, the truth is that honey does not transform entirely into crystalline ice. Boiled honey solidifies faster in a frozen state, and its texture becomes stiffer. This is due to physical changes from the evaporation of water and an increase in sugar concentration.
When honey is boiled above 100 degrees, the water content within it rapidly evaporates. If the original 17% moisture content drops to less than 5-10%, what remains is essentially a high-concentration sugar solution. As the concentration of sugar and water changes, so does the viscosity and freezing properties of the substance.
Generally, liquids with high water content freeze well around 0 degrees in a freezer, but concentrated sugar solutions like honey only begin to freeze at temperatures below -40 degrees. In a household freezer set to -18 degrees, unboiled honey does not freeze well, but boiled honey with almost no moisture becomes very firm quickly. The reason it appears frozen lies here.

The critical difference is not the freezing point of honey but its physical texture. When boiled, the fructose and glucose in honey partially decompose, leading to the caramelization reaction. The color deepens, the viscosity rises, and a texture close to solidification forms. When frozen in this state, it turns into a hard and non-spreadable ‘lump’.
This is not a ‘true freezing’ that forms a crystalline structure like ice, but rather a transition to an amorphous solid state. Water forms crystals as it freezes, but honey solidifies in a random structure. It may shatter like glass but does not have a transparent, hard structure like ice.
Ultimately, the sensation of ‘freezing’ we experience when honey is boiled and frozen is close to an illusion that results from an extremely high viscosity due to decreased moisture and sugar concentration.
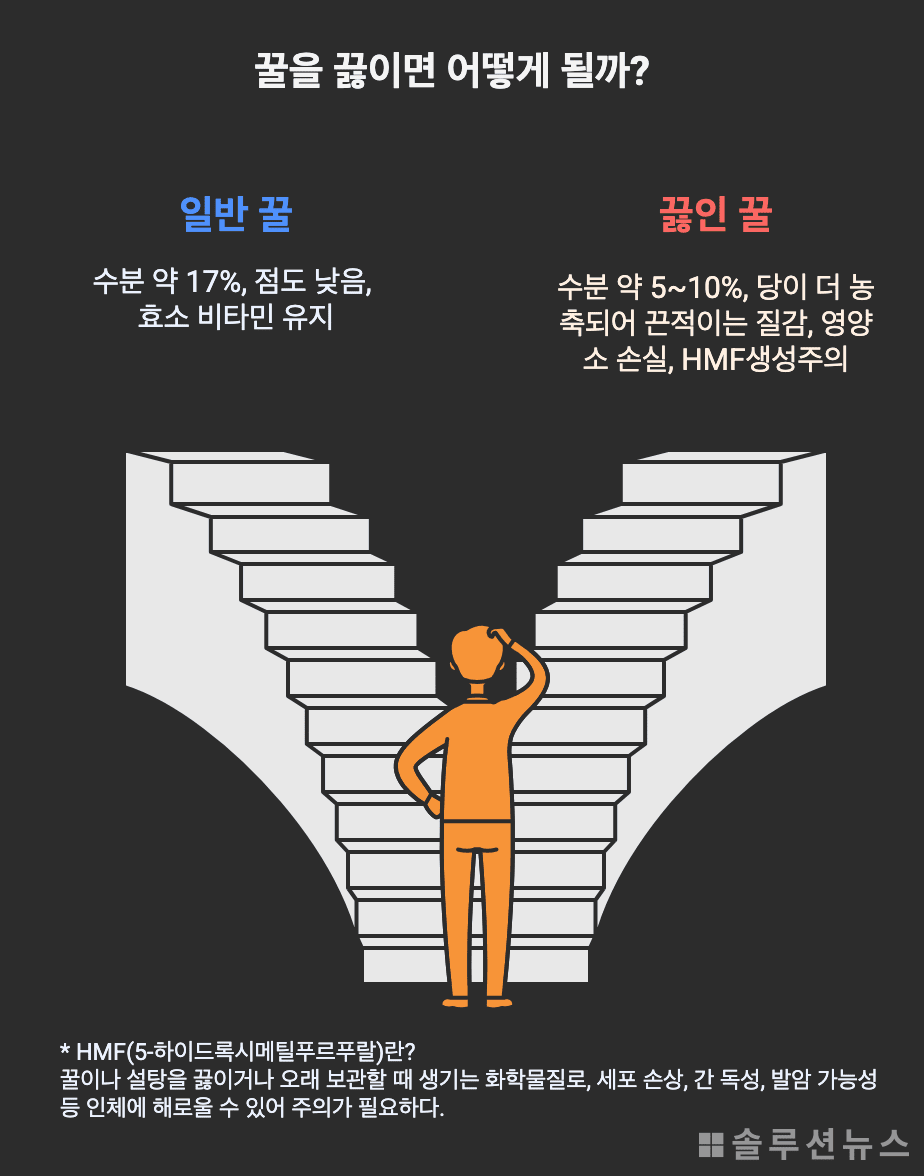
Boiling honey may make it freeze, but the process involves several caveats. First, beneficial enzymes and antioxidants in honey are significantly destroyed during heating. Additionally, a substance called 5-hydroxymethylfurfural (HMF) is produced, which indicates prolonged exposure to high temperatures and may be harmful at high concentrations.
Another issue is the difficulty in handling it. Hot sugar solutions pose a risk of burns, and its sticky and solidifying nature requires careful handling during storage and thawing.
In conclusion, boiling honey can make it harder in texture. However, this is the result of moisture concentration and physical changes rather than freezing effects. Even without boiling, placing honey in the freezer can lead to a sufficiently sticky and thick state, albeit taking a little longer.